
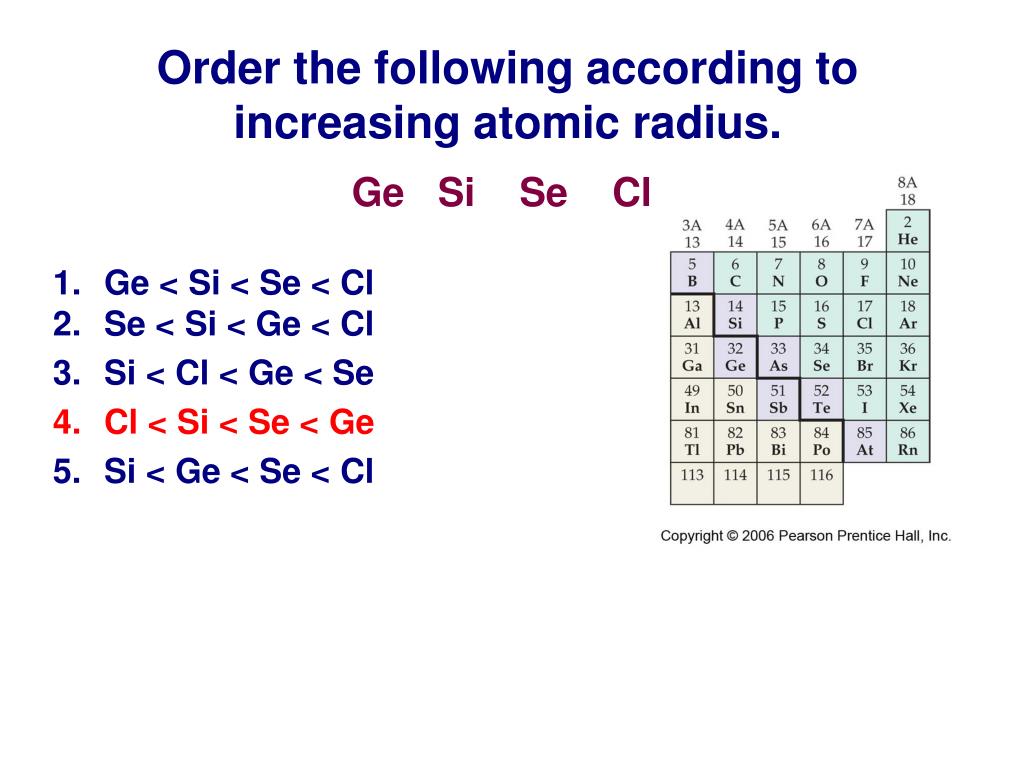
Electron configuration is the arrangement of electrons around the nucleus of an atom based on their energy level. Each successive element adds an energy level. These unbranded versions of the same content are available for you to share, adapt, transform, modify or build upon in any way, with the only requirement being to give appropriate credit to Siyavula. 1 the arrangement of the elements (esabm). 5) Arrange the following atoms in order of increasing radius: N, Sb, P, Bi, As. For more information, visit Creative Commons Attribution-NoDerivs 3.0 Unported.įind out more here about the sponsorships and partnerships with others that made the production of each of the open textbooks possible. Question: Arrange these elements according to atomic radius: Se, Br, Kr, As, Ge, Ga, Ca, K. The CI Ag first is to arrange the elements in a regular series, that AI Ni passes through a zero, according to the empirical equation ,F + ( 4P ) + K W. The general trend is that atomic sizes increase as one moves downwards in the Periodic Table of the Elements, as electrons fill outer electron shells. So as we know that then to be loathing down the group.

And we have to arrange them in increasing order of atomic radius. That is oxygen, sulfur, selenium, thallium. The only restriction is that you cannot adapt or change these versions of the textbooks, their content or covers in any way as they contain the relevant Siyavula brands, the sponsorship logos and are endorsed by the Department of Basic Education. Smallest radius VIDEO ANSWER:Hello student here we are giving us some elements. You can burn them to CD, email them around or upload them to your website.
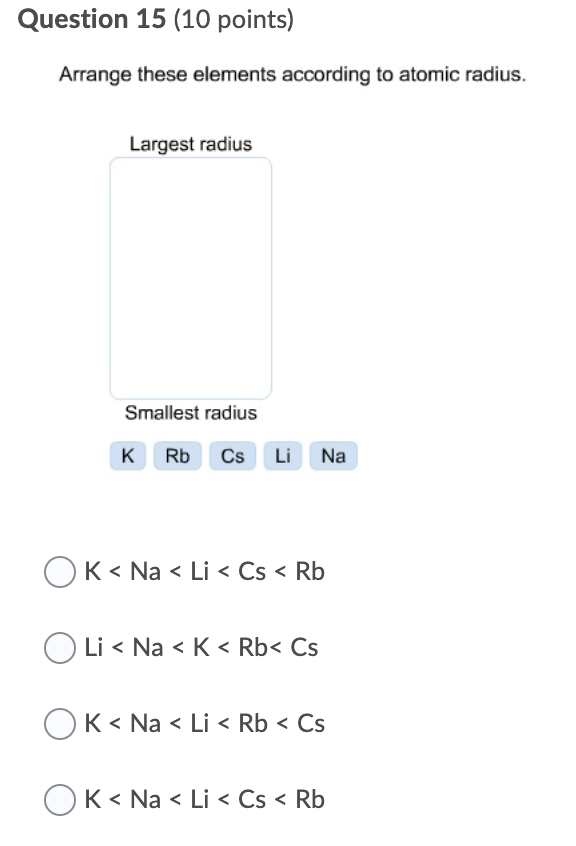
#Arrange these elements according to atomic radius. Pc
You can download them onto your mobile phone, iPad, PC or flash drive. You can photocopy, print and distribute them as often as you like. Silicone will have a greater atomic radius than sulfur, which will have a greater atomic radius than the smallest one chlorine, and phosphorus, which will have a greater atomic radius than the smallest one chlorine. You are allowed and encouraged to freely copy these versions. To place these elements in order of decreasing atomic radius, its going to be in the order.
Better than just free, these books are also openly-licensed! The same content, but different versions (branded or not) have different licenses, as explained: CC-BY-ND (branded versions)


 0 kommentar(er)
0 kommentar(er)
